Effectiveness of Surgical Treatment for Tarlov Cysts a Systematic Review of Published Literature
Introduction
Tarlov cysts (TCs) were start described by Dr. Tarlov in 1938 (1) as perineural cysts filled with cerebrospinal fluid (CSF) that originate from the dorsal ganglion or the spinal posterior nerve root (2). TCs are usually plant in the sacral region, are usually asymptomatic, and normally present as incidental finding (3). Co-ordinate to the literature, about 1% of TCs are symptomatic (4). The symptoms of TCs include depression back hurting, radiculopathy, leg weakness, and paresthesia in the lower limb (5). Magnetic resonance imaging (MRI), computed tomography (CT), or myelography can exist used to confirm TC, with MRI beingness the golden standard modality (6). Electromyography (EMG) can be useful for assessing patients who show symptoms of neurologic changes (iv). There is no consensus on the appropriate treatment of TCs; known treatments for symptomatic TCs include conservative management, percutaneous interventions, or surgical procedures such as laminectomy and fenestration (7). Although endopelvic extension of TCs is uncommon, these cysts may present as an incidental finding on routine gynecological ultrasound imaging (eight). Gynecologists and obstetricians who lack experience with large TCs may often misdiagnose these cysts as adnexal masses.
We report a case in which a TC was misdiagnosed as an adnexal cyst in a 38-year-old adult female (gravida 1, para i). She had visited a local gynecology hospital for a routine health checkup without whatsoever symptoms, and pelvic ultrasonography showed a left 6.51 × 4.96 cm cystic mass (Effigy one). The lesion was an anechoic unilocular cyst with a smoothen thin wall (no internal septations or solid component). The patient was premenopausal and her pregnancy plan was non clear. The cyst was relatively big and had grown in size past ~1 cm over a year. The clinician diagnosed the cyst equally a simple ovarian cyst and planned to remove it. Instead of surgical handling, alcohol sclerotherapy was selected to preserve ovarian function and ensure patient convenience. Sclerotherapy was performed with a fine needle under transvaginal sonographic guidance. Under direct United states guidance, the needle was inserted through the vaginal fornix into the center of the cyst, and the fluid was aspirated. After, 100% ethanol injection and irrigation was conducted using twenty mL of ethanol twice. After sclerotherapy, the patient experienced severe headache when upright, which was accompanied by nausea and airsickness every bit well as bilateral lower leg weakness and radiating hurting in the left leg. Considering her symptoms persisted after i week, she visited a local medical spinal center. Spine MRI showed a 7.ane × five.6 cm cystic mass originating the sacral expanse (Figure 2). Brain MRI was also performed and it showed no abnormal lesions. The patient was treated with medication for hurting command and physical therapy. After conservative treatment, the headache, nausea, and vomiting improved, but the weakness in both legs and radiating pain in the left leg persisted. Ii months after sclerotherapy, the patient first visited the department of physical medicine and rehabilitation at our hospital by walking independently. On the Medical Research Quango manual muscle test, muscular weakness was observed in both lower limbs (grade 3+ on the right side and 3 on the left side; Table 1). She complained of bilateral sensory change beneath the L4 dermatome, manifesting as pain, temperature, vibration, and proprioception. Ankle clonus and Babinski sign were negative. Deep tendon reflex was slightly decreased at the knee and talocrural joint jerk, and the anal tone was decreased. The patient also complained of anal sphincter weakness of 40% and reported symptoms of a neurogenic bladder, namely, incontinence, nocturia, and high urinary frequency; however, self-voiding was possible. To determine the cause of the weakness, pain, and sensory change, and ascertain the severity and localization, nosotros performed electrophysiological examinations. Information technology revealed lower lumbosacral polyradiculopathy and sacral arc dysfunction, clinically cauda equina syndrome (CES) (Tabular array 2). We applied therapeutic modalities to the left leg to save the radiating hurting, and the patient received therapeutic exercises which include lower leg muscle stretching, strengthening, and balancing training. Subsequently half-dozen months, the lower leg weakness, radiating pain in the left leg, urinary incontinence, anal sphincter weakness, and sensory change showed some improvement. Follow-up EMG revealed that the CES was in an incomplete recovery land (Table 2), while follow-upwards spine MRI showed no change in the size 10 months after sclerotherapy (Figure iii). The radiating hurting in the left leg disappeared and muscle strength normalized afterwards 18 months (Tabular array 1). Moreover, the symptoms of neurogenic bladder disappeared and anal sphincter function normalized. On follow-upwards EMG, CES was in nigh full recovery land (Table 2).
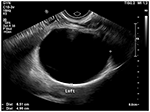
Figure 1. Pelvic ultrasonography shows a left 6.51 × four.96 cm ovarian cyst.
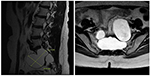
Effigy 2. A sagittal T2-weighted epitome shows a 7.1 × 5.6 cm cyst that originated from the perineurium in the sacrum with extension to the pelvic cavity with bony erosion (left) and the sacrum on the transverse T2-weighted prototype (correct).

Table ane. MRC grades 2, 6, and 18 months later on alcohol sclerotherapy.
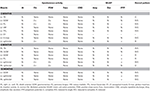
Table ii. Needle EMG findings 2, 6, and 18 months after booze sclerotherapy.
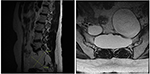
Figure 3. A sagittal T2-weighted epitome shows a 7.1 × five.6 cm cyst that originated from the perineurium in the sacrum with extension to the pelvic cavity with bony erosion (left) and the sacrum on a transverse T2-weighted image (right) obtained 10 months afterwards sclerotherapy.
Materials and Methods
Written informed consent was obtained from the patient for the publication of any potentially identifiable images or data included in this commodity.
Literature Search Strategy
We reviewed all papers that reported TCs mimicking adnexal masses, with the papers identified by searching MEDLINE, EMBASE, SCOPUS, Web of Science, the Cochrane Central Register of Controlled Trials, the Earth Health Organization International Clinical Trials Registry Platform, and the clinical trials registry and database of the U.Due south. National Institutes of Wellness (ClinicalTrials.gov) through October 12, 2020. We placed no restrictions on language or year of publication in our search, and we performed searches using the post-obit keywords: Tarlov cyst, perineural cyst, adnexal mass, sclerotherapy, management, and prognosis.
Nosotros identified 21 patients with TCs mimicking adnexal masses in 12 studies (Table iii). Nosotros included all cases in which the TC was misdiagnosed or considered as an adnexal mass in pelvic sonography, and we excluded cases in which the TC was not misdiagnosed or considered as an adnexal mass.
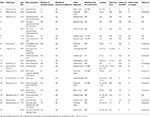
Table 3. Clinical data of patients with TCs mimicking adnexal masses.
Results
We identified 21 cases of TCs mimicking adnexal masses and analyzed the epidemiology, symptoms, initial diagnoses, conditional ultrasound diagnoses, confirmative modalities, sizes, locations, treatments and outcomes in the selected cases.
Epidemiology and Clinical Manifestations
The median patient age was 41 years (range: 26–76 years, mean: 41.three years, standard deviation: 13.04 years). All patients were female person, and sixteen were symptomatic (76%). Pelvic pain (v cases, 24%) (2, 3, 11, 12) and low intestinal hurting (v cases, 24%) (2, 3, thirteen, 14) were the near common symptoms, followed by depression back pain (15) and perineal pain (iii, xiii) (2 cases each, ten%) and constipation (5) and low urinary flow (10) (i case each, 5%). Five patients were asymptomatic (24%) (ii, 6, 8, 9). The duration of symptoms was approximately ane year.
Imaging Report
The initial diagnostic modality was ultrasonography in all cases. The nearly common wrong diagnosis or initial diagnosis was an unspecified adnexal mass (iii, 6, 14). Other diagnoses included presacral mass (5, 8, 11, 13, 15), hydrosalpinges (2, 12), ovarian cyst (2, x), and pelvic mass (9, 13, xv). Confirmative diagnostic modalities were MRI simply (xiv cases, 66.7%) (2, v, viii, 12–15), both MRI and CT (6 cases, 28.6%) (iii, 6, 9, 11, thirteen), or CT merely (i case, 4.8%) (2). All cysts were sacral lesions that showed pelvic extension. The average cyst size was 6.9 cm [range: 5 cm (9, 10) to 11 cm (5), median: six.nine cm, standard difference: 1.46 cm].
Management
The patients were treatments with surgery (7 cases, 33%) (5, viii, ten, eleven, 13, 15), conservative management (4 cases, xix%) (2, 12, fourteen), percutaneous intervention (1 case, five%) (13), booze sclerotherapy (1 case, 5%), and observation (3 cases, 14%) (2, half dozen, 9) (Table 4). Express treatment-related information was available for five cases (24%) (iii).

Tabular array 4. Type of management depending on the presence of symptoms in TCs misdiagnosed as adnexal masses and the clinical form.
V patients were asymptomatic. Amid them, 1 underwent unnecessary surgery (eight), one (the case described above) received alcohol sclerotherapy, and the other three (2, 6, ix) did not receive any treatment (Table iv).
The types of surgery were laminectomy in 3 cases (five, 10, 11), repair of leakage later cystectomy in 3 cases (8, 13, 15), and fistula-blocking surgery in 1 case (15). Conservative treatment consisted of assistants of a nonsteroidal anti-inflammatory drug, physical therapy, and antibiotics. In the 1 case that involved a percutaneous intervention (13), the patient underwent percutaneous drainage and fibrin glue injection. Unusually, one non-symptomatic case was treated with alcohol sclerotherapy (100% alcohol irrigation, 20 mL, immediately repeated twice). The time to intervention or surgery was within i month. In the cases in which symptoms improved after conservative handling, the elapsing of treatment was several months.
Prognosis
Among the three symptomatic patients who underwent laminectomy performed on symptomatic patients, two showed pain relief (5, 11) and one showed no improvement (10). In the patient who underwent fistula-blocking and cyst-filling surgery, the TC shrank and the patient reported relief from symptoms (15). Two (two) of the four patients who received conservative handling showed symptom improvement, one (12) showed no modify in symptoms, and no data was bachelor for the fourth patients (14). The patient who received percutaneous drainage and fibrin glue injection (thirteen) showed symptom improvement within 3 months and cyst shrinkage over 96 months of follow-up (Table four).
In one example (8), an asymptomatic large TC was misdiagnosed equally a presacral mass, and marsupialization was performed with exploratory laparotomy, resulting in leakage of CSF; the patient experienced headache, vomiting, and sixth cranial nerve palsy. A reoperation was performed to ligate the CSF fistula, and the patient'south symptoms fortunately improved. In the other ii symptomatic cases misdiagnosed as pelvic masses (13, fifteen), cystectomy was performed and a CSF leak occurred, requiring repair of the leak; the symptoms improved afterward repair surgery. In our case, incorrect treatment with alcohol sclerotherapy of an asymptomatic large TC mistaken for an adnexal mass caused chemical CES. With conservative treatment, symptoms improved without significant complications after 18 months. Thus, these three previous cases (8, xiii, xv) and our case presented unexpected complications as a effect of mistaking the TC every bit a pelvic mass or adnexal mass.
Discussion
In the nowadays case, the clinician performed alcohol sclerotherapy later a misdiagnosing a TC equally a uncomplicated ovarian cyst. Alcohol sclerotherapy is a transvaginal ultrasound-guided aspiration and ethanol injection technique that is soon used every bit an alternative therapeutic modality for simple ovarian cysts or endometriomas (xvi, 17). The clinician chose alcohol sclerotherapy to preserve ovarian function and to ensure for patient convenience. However, since the patients actually had a TC, not a simple ovarian cyst, the alcohol sclerotherapy caused chemical CES. The anechoic unilocular cyst should have been diagnosed more carefully. In pelvic sonography, the clinician could have checked the separation of the cyst from the ovary, its immobility with respiration, and its connection to the sacrum to place the TC. For more advisable diagnosis and handling, MRI would have been helpful. According to the consensus argument of the Society of Radiologists in Ultrasound, MRI should have been performed, not an immediate treatment (18). However, unfortunately, immediate handling was implemented in this instance. Nevertheless, the patient showed near total recovery without significant complications after 18 months. This clinical class too provides important data regarding the regeneration after alcohol-induced denervation in TC.
Our literature review outlines the importance of familiarizing gynecologists and obstetricians to TCs, which are cystic masses distinct from functional cysts, endometriomas, teratoma, hydrosalpinges, and peritoneal inclusion cysts with similar characteristics (xix). TCs show a tubular/cystic or multilocular/multiseptate appearance in pelvic sonography (2). However, these features may not exist apparent in all cases, and TCs can show diverse manifestations ranging from a simple rounded cyst to a complex loculated cystic mass. Considering TCs can testify various sonographic features, the following aspects should be considered together.
• The size of the TCs that were mistaken for adnexal masses in the literature review was 5 cm or more (mean size: 6.nine cm), while the median age of the population was 41 years. Accordingly, clinicians should consider the possibility of TCs when treating middle-aged women with adnexal cysts that are 5 cm or greater in size.
• Since TCs are extraperitoneal cysts, they do non show mobility during respiration.
• Since TCs are perineural cysts originating from the lumbosacral nerve root, identification of a connexion to the posterior pelvic wall is of import, i.due east., TCs are located posteriorly on the sacrum.
• In contrast to other adnexal cysts, TCs are separated from reproductive organs such as the ovary and salpinx.
• Patients presenting with a symptomatic endopelvic TC may show depression back hurting, sciatica, leg weakness, and other neurologic deficits, so these findings may also serve every bit a point of differentiation.
These differentiating points are summarized in Table 5. TCs can also take internal echoes and appear as slightly elongated, multilocular, or beaded cystic masses posteriorly (12). If an observed cyst is posteriorly located and does not motion with respiration, TCs should be considered along with abscesses, hematomas, endometriomas, and lymph nodes (2). Although an endometriotic cyst with adhesion could evidence low mobility with respiration, extraovarian endometriosis is rarely cystic and, in full general, does non reach a large size (3). TCs appear less elongated and tubular than hydrosalpinges, and the incomplete septation or "waist sign" observed in hydrosalpinges may non be present in a TC (twenty). The presence of ovarian tissue with follicles around the mass will help confirm an ovarian origin (12).

Table 5. Differential diagnosis of benign cystic adnexal masses.
A comprehensive understanding of TCs, including the points of differentiation, begins with a thorough agreement of anatomy. TCs are perineural cysts of the sacral area that arise between the perineurium and endoneurium. In 1938, Dr. Tarlov start reported a TC of the filum terminale as an incidental autopsy finding, and the lesion was classified as a blazon II meningeal cyst by Nabors et al. (21). TCs are cysts filled with CSF and are commonly located most the dorsal root ganglion and tin contain nerve fibers (4). Since their original clarification, TCs have been found all along the spinal nerve roots, not only in the lumbar region (10). 1 report revealed the following incidence rates for perineural cysts at the different spinal levels: cervical level, one.xviii%; thoracic, five.53%; lumbar, 1.05%; and sacral, 15.17% (22). Amidst the cases showing TCs, single anomalies were constitute in 29% and multiple unilateral or less oftentimes bilateral changes were noted in the remaining 71% (22). The prevalence of TCs is estimated to be between ane and 5% of the population, and 20–26% of TCs are thought to be symptomatic, accounting for near i% of the population (iv). The prevalence of TCs increases with age (23). They are significantly more common in women, and women are also more likely to be symptomatic (4). MRI studies of patients with back pain have revealed that 70% of patients with TCs are women, and sexual practice-related differences in the limerick of the dura mater or spinal nerve roots have been postulated to exist the underlying cause of this female person predominance (7).
The pathogenesis of these cysts is unclear, although various hypotheses have been proposed to explain the formation of the slit valve mechanism that allows CSF to pass into the cysts (10). There is however causal bear witness supporting traumatic hemorrhage, pseudomeningoceles, hydrostatic CSF pressures, built diverticula from persistent embryonic fissures, inflammation in the subarachnoid space, inflammation within the nervus root cysts leading to inoculation of fluid, arachnoidal proliferation along and around the exiting sacral nerve root, and hemosiderin deposits breaking down venous drainage in the epineurium and perineurium afterward trauma (four). Consequently, the temporarily increased pressure within the cyst may stretch any overlying nervus fibers within the cyst wall or may compress the ventrally displaced chief portion of the nerve root, which, in plough, may lead to exacerbated radiculopathy or sensory loss, compression of the adjacent sacral thecal sac, and associated urinary and bowel incontinence (24). Symptomatic cysts practice non resolve (25). In a retrospective cohort study of 28 subjects, TCs showed relative growth rates of 2.9 ± 2.6%, four.3 ± 3.8%, and 1.4 ± 1.4% in the anteroposterior, craniocaudal, and transverse dimensions per year, and none of the cysts decreased in size between successive MRI examinations (26). Bone erosion is quite common and is a characteristic feature of large TCs that have grown quickly (5). Although it is unclear whether bone erosion causes bony hurting, rapid growth of the TC may result in bony hurting. In the slit-valve machinery, TCs can grow gradually, and TCs up to 11.three × x.three × 9 cm in size have been reported (5).
Common clinical presentations include low back hurting, sacrococcygeal hurting, leg weakness or pain, sciatica, perianal pain, neurogenic claudication, bowel and bladder dysfunction, and sexual disturbances. TCs can likewise cause unusual clinical symptoms (intestinal or pelvic pain) if the cysts are in the presacral region (thirteen). MRI, CT, or myelography tin be used to confirm the findings of TCs, with MRI existence the gold standard modality (half dozen). TCs are isodense with CSF on noncontrast CT scans and can often be seen to cause various osseous abnormalities and erosions (27). In MRI, TCs showed loftier signal intensity on T2-weighted sequences and low indicate intensity on T1-weighted sequences (12). MRI tin also be used to delineate the exact relationship of the cyst to the thecal sac, besides equally the full volume of fluid inside the cyst (27).
Asymptomatic TCs generally practice not require treatment (27). Conservative treatment, including medical therapy and physical therapy, is suggested as the first-line pick for symptomatic TCs (21). Nonsteroidal anti-inflammatory drugs and neuropathic pain medications have been shown to yield mild comeback in hurting symptoms (iv), and oral steroids have been reported to exist helpful in the treatment of TCs (21). Pelvic physical therapy may assistance alleviate any associated pelvic floor myofascial pain or dysfunction (iv). Epidural steroid injections have also been shown to exist helpful in treating the radiculopathy associated with TCs, and they may be especially helpful in treating the pelvic pain caused past TCs (28). Other intervention options include external CSF drainage, percutaneous cyst drainage, and percutaneous fibrin mucilage injection (5). Fibrin deposition on cyst walls impedes CSF ingress, triggers fibrosis, and, ideally, promotes cyst contracture (29). In the gynecological background, elementary ovarian cysts can be treated using ethanol (sclerotherapy) to destroy the epithelial lining of the fluid-secreting walls, thereby obliterating the cyst crenel and preventing the re-accumulation of fluid (16). The guidelines for sclerotherapy for adnexal cysts accept not been established. Alcohol is neurotoxic and has neurolytic effects that can pb to nerve impairment, and therefore should non be injected into TCs. Nevertheless, booze sclerotherapy does not cause permanent irreversible or complete nerve impairment. In such cases, a serial nervus conduction study and EMG can provide much information almost neural regeneration. Patients with cysts >i.5 cm in size and radicular hurting or bowel/bladder dysfunction have been reported to benefit from surgery (5). Surgical treatment of symptomatic perineural cysts, which involves complete cyst removal and excision of the affected posterior root and ganglion, was advocated by Tarlov and has since been used by others (30). Surgical options include insertion of cyst–subarachnoid, cyst–peritoneal, or lumboperitoneal shunts; simple decompressive laminectomy; resection of the cyst neck; cyst wall resection; cyst imbrication; or bipolar cauterization to compress the size of the cyst (5). One study suggested a elementary and effective procedure with the cardinal step of blocking the inlet of the fistula from inside the dural sac, which is more applicable and minimizes the probability of cyst recurrence (fifteen). The complications of interventional or surgical handling for TCs can exist quite pregnant, including cognitive fat embolisms, positional headaches, CSF leaks, hygienic meningitis, postoperative pseudomeningoceles, and damage to the sacral nervus roots, with resultant lower motor neuron bladder or bowel dysfunction (four). As such, interventional or surgical handling should be advisedly considered.
Our study had a few limitations. The chief limitation was the lack of cases, with but 21 relevant cases identified in the literature search. Although misdiagnosed TCs for adnexal masses are non common, we performed a search for all clinical studies of TCs that mimicked adnexal masses. Secondly, the data were obtained retrospectively. Thus, additional research is needed to prevent misdiagnosis and enable more than accurate diagnosis and handling in the future.
Conclusions
Our data indicates the importance of considering the possibility of a large TC when assessing adnexal masses on sonography. Since TCs tin masquerade as pelvic masses, if the masses appear tubular/cystic or multilocular/multiseptate, practise not move with respiration, and originate from the sacrum in sonography with or without neurologic symptoms, TC should exist considered. Our example is the first to report chemic CES caused by alcohol sclerotherapy for a TC that was incorrectly diagnosed as an adnexal mass. Nonetheless, the patient recovered almost completely without significant complications after 18 months. Accurate diagnosis tin can preclude incorrect medical management and reduce patient discomfort.
Data Availability Statement
The original contributions presented in the written report are included in the article/supplementary materials, further inquiries can be directed to the corresponding author/southward.
Ethics Statement
Written informed consent was obtained from the private(s) for the publication of whatever potentially identifiable images or data included in this article.
Author Contributions
SK and TK: data analysis, information estimation, literature search, and writing of the manuscript. HL and JP: conceptualization, methodology, and writing of the manuscript. KN: supervision, project assistants, and review of the manuscript. All authors contributed to the article and approved the submitted version.
Funding
This piece of work was supported by the National Enquiry Foundation of Korea (NRF) grant funded past the Korea government (MSIP; Ministry of Scientific discipline, ICT & Futurity Planning) (No. 2019R1F1A1053196).
Conflict of Interest
The authors declare that the research was conducted in the absenteeism of any commercial or financial relationships that could be construed equally a potential conflict of interest.
References
1. Tarlov I. Perineural cysts of the spinal nerve roots. Curvation Neurol Psychiatry. (1938) forty:1067–74. doi: ten.1001/archneurpsyc.1938.02270120017001
CrossRef Full Text | Google Scholar
3. McClure MJ, Atri Grand, Haider MA, Potato J. Perineural cysts presenting as circuitous adnexal cystic masses on transvaginal sonography. AJR Am J Roentgenol. (2001) 177:1313–8. doi: 10.2214/ajr.177.half dozen.1771313
PubMed Abstruse | CrossRef Total Text | Google Scholar
7. Hulens One thousand, Rasschaert R, Bruyninckx F, Dankaerts West, Stalmans I, De Mulder P, et al. Symptomatic Tarlov cysts are oftentimes overlooked: 10 reasons why-a narrative review. Eur Spine J. (2019) 28:2237–48. doi: 10.1007/s00586-019-05996-1
PubMed Abstract | CrossRef Full Text | Google Scholar
8. Maleci A, Bianco F, Onnis Yard, Di Lorenzo N. Iatrogenic spinopelvic cerebro-spinal fluid fistula. Case report. J Neurosurg Sci. (1995) 39:261–3.
PubMed Abstruse | Google Scholar
9. Raza S, Klapholz H, Benacerraf BR. Tarlov cysts: a cause of complex bilateral adnexal masses on pelvic sonography. J Ultrasound Med. (1994) 13:803–v. doi: 10.7863/jum.1994.13.10.803
PubMed Abstract | CrossRef Full Text | Google Scholar
11. Wang B, Moon SJ, Olivero WC, Wang H. Pelvic hurting from a giant presacral Tarlov cyst successfully obliterated using aneurysm clips in a patient with Marfan syndrome. J Neurosurg Spine. (2014) 21:833–half dozen. doi: 10.3171/2014.8.SPINE148
PubMed Abstract | CrossRef Full Text | Google Scholar
12. Ahmadi F, Akhbari F. Adnexal masses or perineural (tarlov) cysts? Differentiation by imaging techniques: a instance report. Int J Reprod Biomed. (2017) 15:589–92. doi: 10.29252/ijrm.fifteen.ix.589
CrossRef Full Text | Google Scholar
xiii. Wang B, Pu F, Wu Q, Zhang Z, Shao Z. Presacral Tarlov cyst equally an unusual crusade of abdominal pain: new case and literature review. Earth Neurosurg. (2018) 110:79–84. doi: x.1016/j.wneu.2017.10.135
PubMed Abstract | CrossRef Full Text | Google Scholar
14. Boukobza G, Roussel A, Fernandez-Rodriguez P, Laissy JP. Giant multiple and bilateral presacral Tarlov cysts mimicking adnexal mass - imaging features. Int Med Case Rep J. (2018) xi:181–4. doi: 10.2147/IMCRJ.S147791
PubMed Abstruse | CrossRef Full Text | Google Scholar
xv. Zhu H, Shen L, Chen Z, Yang Grand, Zheng X. Giant Tarlov cysts with rare pelvic extension: report of 3 cases and literature review. Globe Neurosurg. (2020) 139:505–eleven. doi: 10.1016/j.wneu.2020.04.112
PubMed Abstract | CrossRef Total Text | Google Scholar
16. Castellarnau Visus 1000, Ponce Sebastia J, Carreras Collado R, Cayuela Font Eastward, Garcia Tejedor A. Preliminary results: ethanol sclerotherapy subsequently ultrasound-guided fine needle aspiration without anesthesia in the direction of simple ovarian cysts. J Minim Invasive Gynecol. (2015) 22:475–82. doi: 10.1016/j.jmig.2014.12.158
PubMed Abstract | CrossRef Total Text | Google Scholar
17. Hsieh CL, Shiau CS, Lo LM, Hsieh TT, Chang MY. Effectiveness of ultrasound-guided aspiration and sclerotherapy with 95% ethanol for treatment of recurrent ovarian endometriomas. Fertil Steril. (2009) 91:2709–thirteen. doi: 10.1016/j.fertnstert.2008.03.056
PubMed Abstract | CrossRef Full Text | Google Scholar
18. Levine D, Brownish DL, Andreotti RF, Benacerraf B, Benson CB, Brewster WR, et al. Management of asymptomatic ovarian and other adnexal cysts imaged at US: society of radiologists in ultrasound consensus conference statement. Radiology. (2010) 256:943–54. doi: ten.1148/radiol.10100213
PubMed Abstract | CrossRef Full Text | Google Scholar
nineteen. Expert Panel on Women's Imaging, Atri M, Alabousi A, Reinhold C, Akin EA, Benson CB, et al. ACR appropriateness criteria® clinically suspected adnexal mass, no acute symptoms. J Am Coll Radiol. (2019) 16:S77–93. doi: x.1016/j.jacr.2019.02.011
PubMed Abstract | CrossRef Total Text | Google Scholar
21. Lucantoni C, Than KD, Wang AC, Valdivia-Valdivia JM, Maher CO, La Marca F, et al. Tarlov cysts: a controversial lesion of the sacral spine. Neurosurg Focus. (2011) 31:E14. doi: 10.3171/2011.9.FOCUS11221
PubMed Abstract | CrossRef Full Text | Google Scholar
22. Burdan F, Mocarska A, Janczarek K, Klepacz R, Losicki M, Patyra G, et al. Incidence of spinal perineurial (Tarlov) cysts among Due east-European patients. PLoS One. (2013) eight:e71514. doi: 10.1371/periodical.pone.0071514
PubMed Abstract | CrossRef Full Text | Google Scholar
23. Kuhn FP, Hammoud S, Lefevre-Colau MM, Poiraudeau South, Feydy A. Prevalence of simple and complex sacral perineural Tarlov cysts in a French accomplice of adults and children. J Neuroradiol. (2017) 44:38–43. doi: 10.1016/j.neurad.2016.09.006
PubMed Abstract | CrossRef Full Text | Google Scholar
24. Sharma M, SirDeshpande P, Ugiliweneza B, Dietz N, Boakye M. A systematic comparative outcome analysis of surgical versus percutaneous techniques in the management of symptomatic sacral perineural (Tarlov) cysts: a meta-assay. J Neurosurg Spine. (2019) xxx:623–34. doi: x.3171/2018.ten.SPINE18952
CrossRef Full Text | Google Scholar
26. Yang AI, Rinehart CD, McShane BJ, Hitti FL, Welch WC. Growth of lumbosacral perineural (Tarlov) cysts: a natural history analysis. Neurosurgery. (2020) 86:88–92. doi: 10.1093/neuros/nyy586
CrossRef Full Text | Google Scholar
27. Acosta FL Jr, Quinones-Hinojosa A, Schmidt MH, Weinstein PR. Diagnosis and management of sacral Tarlov cysts. Case study and review of the literature. Neurosurg Focus. (2003) 15:E15. doi: x.3171/foc.2003.xv.2.15
PubMed Abstruse | CrossRef Full Text | Google Scholar
29. Murphy One thousand, Oaklander AL, Elias G, Kathuria South, Long DM. Handling of 213 patients with symptomatic Tarlov cysts by CT-guided percutaneous injection of fibrin sealant. AJNR Am J Neuroradiol. (2016) 37:373–9. doi: 10.3174/ajnr.A4517
PubMed Abstract | CrossRef Full Text | Google Scholar
30. Elsawaf A, Awad TE, Fesal SS. Surgical excision of symptomatic sacral perineurial Tarlov cyst: case series and review of the literature. Eur Spine J. (2016) 25:3385–92. doi: 10.1007/s00586-016-4584-iii
PubMed Abstract | CrossRef Total Text | Google Scholar
Source: https://www.frontiersin.org/articles/10.3389/fmed.2020.577301/full
0 Response to "Effectiveness of Surgical Treatment for Tarlov Cysts a Systematic Review of Published Literature"
Post a Comment